hii can any person suggest how we could outsource purifies water and what doc Now we have to get ready for it
5. Prior to the end of period I, system is initiated to work with a few stress or tense conditions like get started of system following failure of electricity or start up following unexpected emergency system shut down. System is simulated to operate beneath typical scenario of maintenance like start up of system right after regeneration of resin, filter changing, ozone generator failure etc. & in the final water system (Standard Operating Process )SOP’s developed.
A very good revalidation process is depending on the initial validation And the way it prepared to redress many improvements inside the system. The preliminary validation approach need to be robust enough to give a transparent difference between A serious and also a small adjust.
Take a look at procedures needs to be created in a method that is definitely total, understandable and probable to repeat. With all qualifications, it's important to collect all relevant details, make clear references to paperwork applied, mark attachments and evaluation carried out exams with regards to completeness, traceability and signatures.
Your browser isn’t supported anymore. Update it to have the greatest YouTube practical experience and our most up-to-date capabilities. Find out more
The DQ document ought to check here cover all the mandatory diagrams, Structure, area Suitability wished-for special element of factors, products and their specification, desired substance of construction, area with the control panel, electrical prerequisite and utility necessity
Even if the design of water therapy systems is currently normally standardised and reproducible, Specific circumstances do occur in apply.
The contents of ISPE’s steering documents, equally printed and electronic, are safeguarded by legislation and meant solely for the non-public non-professional usage of the person purchaser.
Feedback should be specified in producing and compiled in a single doc clarifying that has commented on what. For quickly-track jobs, these more info acceptance routines are particularly critical and has to be set up in the beginning in the task. It's also suggested that the number of approving functions is stored to a bare minimum. The person ought to specify which regime applies to change requests inside the venture and from when it's applicable.
“We’re executing the ideal we can easily simply because our families live in the Neighborhood also, and we treatment about people today’s health and welfare,” Lyons stated. “We’re resolving challenges on a daily basis and there’s a whole lot to be good about.”
The period of Process Qualification (PQ) for a water system will depend on several variables, including the complexity on the system, the criticality on the water good quality for the process, and regulatory requirements.
Water is important to pharmaceutical processing: present as an excipient; used for reconstitution of products; throughout synthesis; throughout production of the concluded products; to be a cleansing agent for rinsing vessels, devices, Principal packaging components; and for the dilution of disinfectants.
Facility qualification shall be initiated after the protocol for facility qualification is approved & signed.
six. All of the managing devices must be calibrated and Licensed as per published procedures that they are precise, specific, selective and particular.
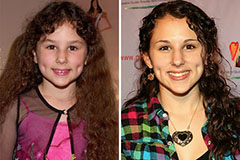 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Kelly McGillis Then & Now!
Kelly McGillis Then & Now! Val Kilmer Then & Now!
Val Kilmer Then & Now! David Faustino Then & Now!
David Faustino Then & Now! Lacey Chabert Then & Now!
Lacey Chabert Then & Now!